Services
-
Developability Assessment
-
TOP clone screening
(including stability evaluation)
-
High-throughput screening of culture media
(domestic and imported)
-
Customized process optimization
(Yield improvement & quality adjustment)
-
Fed-batch process development
-
Perfusion technology based process development
-
Process transfer and scale-up
Strengths and Capabilities
Rapid development
Start the process development from mini-pool;
Process development and locked, from shake flask to reactor can be completed in 8-12 weeks.
Excellent quality
Following the QbD concept and considering production cost, developing a stable, high yielding and competitive cell culture process;
Adhering to GLP principles, regulating the management of all nodes in the development process. For example, the authenticity and completeness of experimental records, the scientific and logical nature of report writing, etc.
Diversified and Scientific Process Development based on perfusion technology
Mastering more Know-How to continuously expand and improve upstream process development including control strategies and in process control, etc.
Demonstration Case
Customer 1
Challenges:
There
are obvious growth differences between shake flask and bioreactor processes in
PD stage; tech-transfer to pilot scale, it difficult to control such as lactic
acid accumulation, low yield and high mannose in N-glycan; tight development
time.
Strategy:
-
High-throughput medium screening;
-
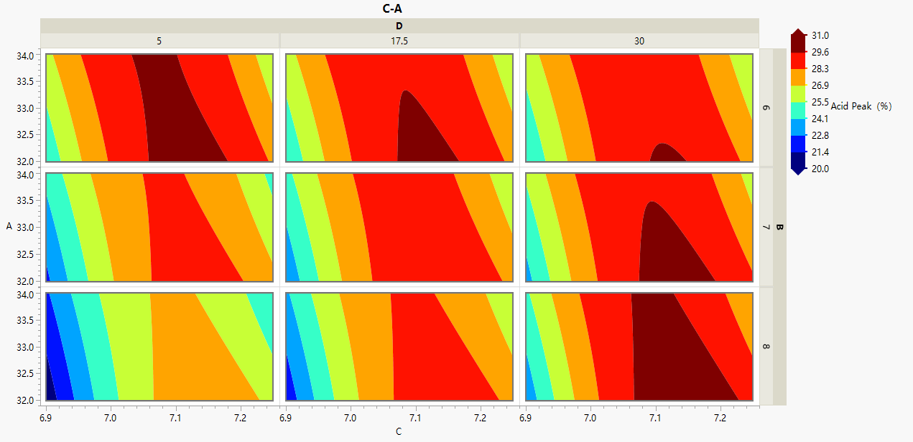
DOE experimental design is used
in the shake flask process development stage to identify key factors that can
increase yield and reduce Man5 content;
-
Cultivation parameters of
bioreactor were explored synchronously, and the critical process parameters
which affect cell growth were determined and scale-up successfully.
-
DOE experimental results (JPM software analysis)
-
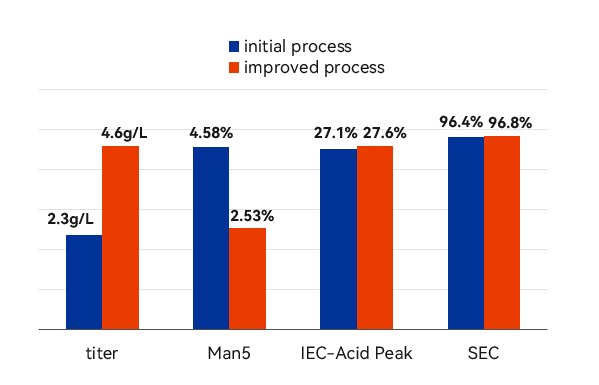
Process optimization results
-
-
Result
– Yield increased significantly to 4.5 g/L, Man5 content reduced to 3% and good batch consistency. Tox and GMP batch production have been completed and are consistent with the process development.
Customer 2
Challenges:
Domestic substantiation; production increase; Keep quality consistency basically.
Solutions
-
Medium screening
-
Adjusting glycoform using platform method
-
BR’s
parameters
optimization
Process Optimization Results

Result
– Medium had localized and yield increased to 4.2 g/L, quality data were within the acceptable range, 3L was consistent with 200L.
Workshop
