Drug Substance Manufacturing

Cell banking

Pilot-scale manufacturing

Commercial manufacturing
BioDlink has established a commercial GMP production base for biological drugs, which is equipped with a series of cell bank production lines (independent workshops) and several independent drug substance production lines. We provide MCB/WCB production and antibody DS manufacturing services in various scales, from 200 L to 2,000 L, with total capacity >20,000 L.
– BioDlink uses production equipment of international leading brand, including Cytiva’s single use bioreactors and chromatography systems, as well as Millipore and PALL’s ultrafiltration systems.
– With single use technology and highly flexible production strategy, stages for batches and specifications at the same time can be accommodated.
– With extensive experience, the team has completed dozens of drug substance production, including the successful completion of multiple batches of marketed products.
Demonstration Case
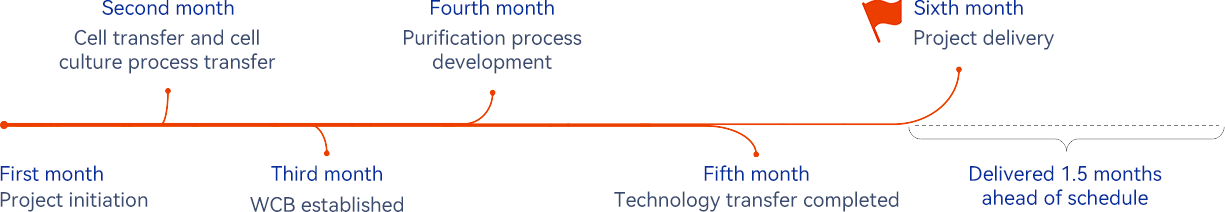
Project highlights
Validated scale-up process
Executable and successful scale up strategy from 3 L to 2,000 L commercial production.
Precise solutions
High efficient execution
The project has been completed ahead of schedule, which includes 18 batches for small-scale study, 9 method transfers, and 4 method development and validation works.
Key Figures Regarding Commercial Manufacturing
Workshop
Disposable dispensing system with weighing module
Automated TFF ultrafiltration system
Automated liquid chromatography systems with chromatography columns