TOT BIOPHARM is
pleased to announce it has received accreditation from Japan’s Pharmaceuticals
and Medical Devices Agency (PMDA) as a Foreign Manufacturer, specifically for
“Biological Products.” This achievement marks a new chapter in the company's
efforts to expand its Contract Development and Manufacturing Organization
(CDMO) services in Japan.
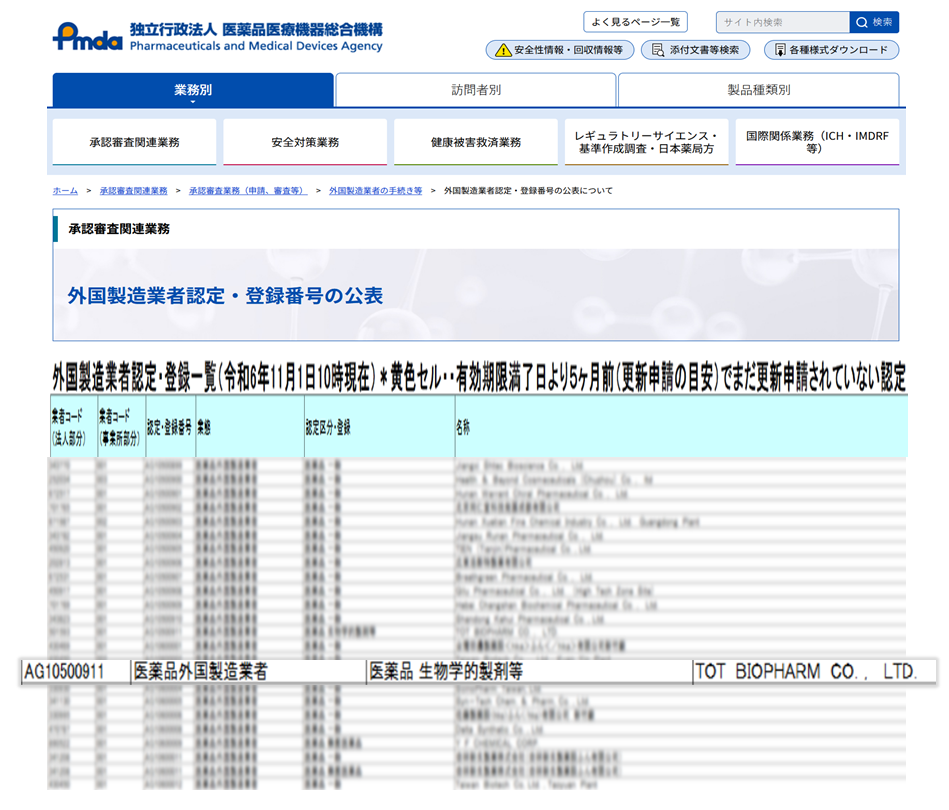
The PMDA's Foreign Manufacturer
Accreditation is a key regulatory requirement for overseas companies seeking to
sell drugs, active pharmaceutical ingredients (APIs), or medical devices in
Japan. Under Japan’s Pharmaceutical and Medical Device Act, all foreign
manufacturers seeking to market drugs or medical products in Japan must obtain
PMDA accreditation. The process involves a comprehensive review of
manufacturing facilities and key personnel to ensure compliance with Japan’s
regulatory requirements. With this accreditation, TOT BIOPHARM has demonstrated
that its facilities meet the required standards, paves the way for the company to
offer high-quality CDMO services in the Japanese market.
TOT BIOPHARM’s quality system already comply
with the regulatory standards set by the U.S. FDA, and the European Medicines
Agency (EMA), and China’s NMPA. Successfully passing the PMDA’s rigorous
evaluation further reinforces the company's ability to provide GMP-compliant
CDMO services in Japan. This milestone is expected to help TOT BIOPHARM provide
stable, high-quality services to the Japanese market and strengthens the
company’s position in its global expansion efforts.
“We are thrilled to have received PMDA
Foreign Manufacturer Accreditation”, said the company management team. “This
qualification opens up exciting new opportunities for us in Japan, allowing us
to enter the market and offer high quality CDMO services. Looking ahead, we
will continue to strengthen our global presence through professional R&D
and production teams, robust production and quality management systems, and a
commitment to meeting the needs of our international customers.”